Trovagene (TROV) Stock: Providing New Cancer Treatment Options With Onvansertib
 We all know what cancer is. The debilitating, often life-threatening condition can be incredibly difficult to treat. As a result, new therapies are being developed in an effort to bring improved treatment options to patients, especially for some of the toughest-to-treat cancers. That’s where Trovagene (Nasdaq: TROV) comes in with their drug candidate, Onvansertib.
We all know what cancer is. The debilitating, often life-threatening condition can be incredibly difficult to treat. As a result, new therapies are being developed in an effort to bring improved treatment options to patients, especially for some of the toughest-to-treat cancers. That’s where Trovagene (Nasdaq: TROV) comes in with their drug candidate, Onvansertib.
Trovagene caught my attention after the company released data from a Phase 1b/2 clinical trial of Onvansertib for the treatment of patients with Acute Myeloid Leukemia (AML). Through the ongoing Phase 1b segment of the trial, the company is working to identify the maximum-tolerated dose of Onvansertib in patients with AML who are ineligible for induction therapy or who have relapsed or refractory disease. The recently-released data proved to be very promising, not just from a safety and tolerability perspective, but importantly, there are signs that Onvansertib, in combination with standard-of-care chemotherapy, may be benefiting patients, based on the preliminary anti-leukemic activity they are seeing in the dose escalation phase of this trial.
What Is Onvansertib?
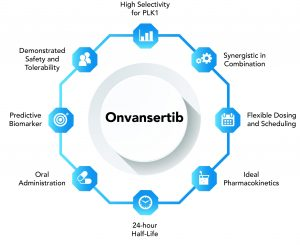
Onvansertib is a first-in-class, 3rd generation, oral and highly-selective Polo-like Kinase 1 (PLK1) inhibitor. There are a few key attributes that set Onvansertib apart from other drugs used to treat patients with AML today…
Orally Administered
Onvansertib is a capsule that is taken orally once a day, so it is easy and convenient for patients.
Relatively Short Half-Life
The half-life of Onvansertib is only 24 hours. This may prove to be an important attribute, as the short half-life may reduce the potential toxicity and adverse events in patients that receive the treatment. To-date, only mild to moderate side effects have been reported.
Highly Selective for PLK1
Onvansertib inhibits PLK1. This is a master-regulator of cell proliferation and is over-expressed in a wide range of cancer types, including leukemias, lymphomas and solid tumor cancers. Through the inhibition of PLK1, Onvansertib causes mitotic arrest in prometaphase, ultimately leading to tumor cell death.
Synergistic in Combination
Onvansertib has demonstrated synergy when combined with numerous chemotherapies and targeted therapeutics, which makes it attractive for use across a number of types of cancer where it can be combined with already approved drugs. What this means is that when you combine Onvansertib with other drugs to treat cancer, the total effect of the drugs is greater than the sum of the individual effects of each drug. TROV is targeting cancer indications where there is a significant medical need to provide patients with new treatment options.
One of the indications being targeted is Acute Myeloid Leukemia, also known as AML. AML tumors are liquid tumors and are incredibly difficult to treat. In fact, there are approximately 20,000 new cases every year, with 10,400 deaths occurring annually and a 5-year survival rate of about 25%. That means only 1 out of every 4 patients diagnosed with the condition will live for a period of five years or longer. Additionally, for patients with relapsed or refractory AML, there are limited, if any, effective treatments currently available. All things considered, there is an overwhelming medical need for new treatment options to treat patients with AML.
The Recently Released Data
At the recent American Society of Hematology (ASH) conference, TROV announced positive safety and preliminary efficacy data from the Phase 1b/2 clinical trial of Onvansertib for the treatment of patients with AML who are not eligible for induction therapy or who have relapsed or refractory disease. While we will briefly review all of the data presented, I wanted to start with a patient case from the trial that demonstrates the potential value of this new targeted therapeutic.
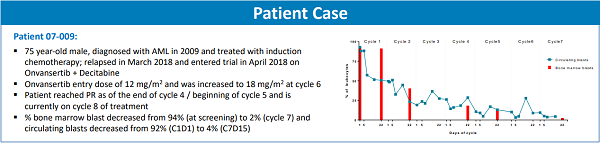
The patient is a 75-year-old male who was first diagnosed with AML back in 2009 and treated with induction chemotherapy. In March of 2018, the patient relapsed, and he was enrolled in the Onvansertib clinical trial in April of 2018. When he first met with his hematologist/oncologist Dr. Amer Zeidan, the lead investigator on the trial, this patient was so ill that he was wheelchair-bound, finding mobility nearly impossible.
In the clinical trial, the patient was treated with Onvansertib, at an entry dose of 12 mg/m2 in combination with Decitabine. At the beginning of his sixth treatment cycle, the Onvansertib dose was increased to 18 mg/m2. To date, the patient continues on treatment, now in his eighth cycle. By the end of the fourth cycle, he had achieved a partial response – the percentage of leukemic blast cells in the bone marrow had decreased from 94%, at the time of screening, to 2% by cycle 7. Correspondingly, leukemic blast cells in the blood also decreased from 92% to 4%. This is a significant response to treatment. Importantly, he has also tolerated treatment with Onvansertib very well.
We often tend to focus on the data from a binary perspective in articles like this one, forgetting about one of the most important outcomes of any treatment. That outcome is the quality of life for the patient. Treatment in Trovagene’s AML trial has made a meaningful difference on his quality of life. No longer too weak and ill to require a wheelchair, he is walking into the clinic for his visits with Dr. Zeidan, smiling and interacting with everyone, and perhaps most telling is that he has been able to resume meaningful activities, including attending an annual golf tournament during the summer.
About The Trial Design And Key Objectives
In Phase 1b, the primary objective is to assess the dose limiting toxicities (DLTs), determine the maximum-tolerated dose (MTD) of Onvansertib and identify the recommended Phase 2 dose (RP2D). The Phase 2 segment of the trial will continue to assess the safety and tolerability of Onvansertib and will also evaluate the clinical activity of the combination regimen. TROV is also evaluating pharmacodynamic and diagnostic biomarkers associated with response to treatment to help identify patients who are more likely to respond to treatment.
The patients being included in the trial are either deemed ineligible to receive induction therapy of they have relapsed or refractory AML. Albeit still early in the trial, Onvansertib in combination with either low-dose cytarabine (LDAC) or Decitabine, may prove to be a new treatment option for these patients, who have few, if any, viable options currently available.
The Data Is Encouraging
The data presented by TROV is encouraging in many respects. Here’s what we saw:
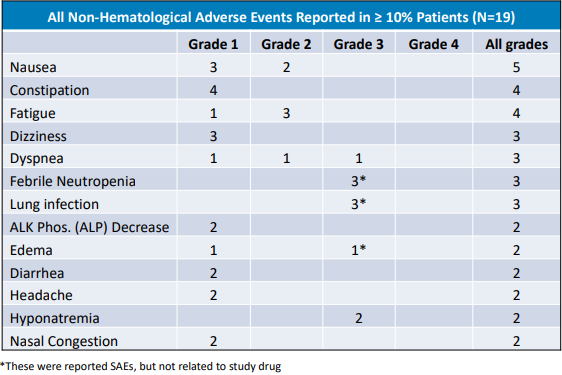
So far, Onvansertib is proving to be safe and well-tolerated among the patient population in the trial. To date, there have been no therapy-related deaths, or therapy-related serious adverse events. In fact, the data indicate that the only adverse event possibly related to Onvansertib was Grade 1 nausea in 4 patients. The table below lists all adverse events that have been reported to-date from the ongoing trial. However, it is important to note that most events are considered to be unrelated to Onvansertib.
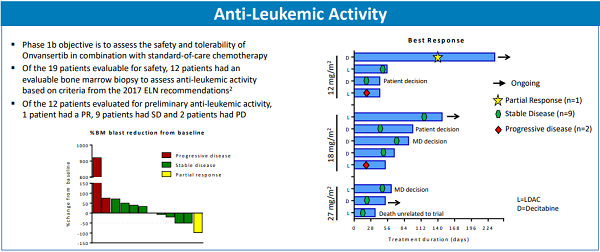
Although the Phase 1b segment of the trial is focused primarily on assessing the safety of Onvansertib, it is encouraging to see the early data associated with anti-leukemic activity, which will be more of the focus in the Phase 2 portion of the trial. Of the 19 patients that were evaluable for safety, 12 had an evaluable bone marrow biopsy to assess anti-leukemic activity. Here’s a quick breakdown of the anti-leukemic assessment that was provided in the recent poster presentation at the 60th American Society of Hematology (ASH) Annual Meeting:
- Partial Response – A partial response has been achieved in one patient (the 75-year-old patient that was described above). This is confirmed by the significant decrease in both circulating and bone marrow blast cells.
- Stable Disease – 9 of the 12 patients achieved stable disease. Stable disease means that since patients started treatment, there has not been progression in leukemic activity.
- Progressive Disease – Finally, 2 of the 12 patients showed progressive disease. This means that, while treatment was provided, their disease has continued to progress, and thus treatment on the trial was discontinued. It’s important to keep in mind that it’s likely these patients were on sub-therapeutic doses, as the Phase 1b is a dose escalation trial and the dose level that will be considered the recommended Phase 2 dose has not yet been reached.
All in all, this data is very promising. It shows that, in one of the most difficult to treat liquid tumor cancers – AML. A benefit has been demonstrated in more than 80% of the patients treated with Onvansertib in combination with LDAC or Decitabine.
All in all, the data demonstrates that not only is Onvansertib proving to be safe and well-tolerated, the combination treatment is showing anti-leukemic activity, albeit it is still early in the trial. With a greater than 80% patient benefit, it appears that TROV may be onto something here.
Breaking Through The Data
Let’s step back for a minute and think about what TROV has achieved here. Onvansertib is in the early stages of clinical development, but even in these early days, the drug is showing great promise.
Because AML is so difficult to treat, there are many patients for whom there are few, to no, effective options for treating their cancer. However, Onvansertib data show that >80% of patients treated to-date have experienced a favorable response to the drug. While TROV is still early in the development of Onvansertib, this data is encouraging.
Onvansertib May Have Potential Across a Number of Cancer Types
Throughout the conversation today, we’ve talked about how Trovagene’s Onvansertib could be changing how we manage the treatment of patients with relapsed or refractory AML. In addition to AML, TROV is also evaluating Onvansertib in combination with Zytiga® (abiraterone acetate)/prednisone in a Phase 2 trial in patients with metastatic Castration-Resistant Prostate Cancer (mCRPC). As in the case of AML, Onvansertib has demonstrated synergy when used in combination with Zytiga® and the goal of this trial is to stabilize disease in patients who are showing early signs of disease progression while taking Zytiga®. As a third indication, TROV is planning to initiate a Phase 1b/2 trial of Onvansertib in combination with FOLFIRI + bevacizumab in 2019, as second-line therapy for patients with metastatic Colorectal Cancer (mCRC) who have a Kras mutation. Tumor biomarkers drive therapy decisions for first-line mCRC therapy and approximately 50% of mCRC is RAS mutant (KRAS). There is a large unmet need in RAS-mutant mCRC as there are currently no targeted therapies available and second-line treatments have only a 5% response rate. This is another cancer indication where Onvansertib has demonstrated synergy when combined with the current standard-of-care regimen.
However, it’s important to remember that these are only three of what may prove to be a number of potential cancers and indications where Onvansertib, which you can think of as a “pipeline within a molecule” may have the potential to provide new and beneficial treatment options for patients.
The PLK1 enzyme, the enzyme that Onvansertib is designed to inhibit, is present in various breast cancers, lung cancers, lymphomas, leukemias, melanoma, and other cancer types. Should TROV demonstrate the benefit of Onvansertib in AML, metastatic prostate cancer and colorectal cancer, all difficult to treat cancers, treatment may also prove to be effective across a number of other cancers and indications.
The Takeaway
The takeaway here is a relatively simple one. TROV may be onto something with Onvansertib, a first-in-class, 3rd generation, oral and highly-selective Polo-like Kinase 1 (PLK1) inhibitor. While still early in their clinical development program, Onvansertib, as part of a combination regimen with already approved drugs, is showing clinical promise and appears to be safe and well-tolerated in patients treated to-date. TROV is working with leading investigators at premier institutions throughout the U.S. We will be watching for more good news and exciting data from the ongoing clinical trials in the coming months.
Note: This article was contributed to Modest Money by Josh Rodriguez. Joshua Rodriguez is the owner and founder of CNA Finance. He is also a partner at Modest Money. His analysis has been featured on Investing.com, Yahoo! Finance, Google Finance, Google News, and many others. This article was originally published with all relevant disclosures at CNA Finance.
Category: Cheap Stocks



